Sick Bees: Part 7 – Transgenerational Immune Priming
Contents
Trans Generational Immune Priming. 1
What is RemebeeTm and How Does it Work?. 4
Natural Amplification and Spread. 4
Results from my California Trial (continued) 5
Sick Bees—Part 7
How does immunity to a virus spread throughout the hive? And can we use this mechanism to our advantage?
Randy Oliver
ScientificBeekeeping.com
First published in American Bee Journal March 2011
Trans Generational Immune Priming
The honey bee does not take the insults thrown at them by all these weird recombinant and chimerical viruses lying down, or we simply wouldn’t have any bees left! As I explained in the last installment, bees can “fix” immunity to a certain virus in their genome. This process has been confirmed with respect to Israeli Acute Paralysis Virus. Since only a percentage of the bee population studied carries the viral genes that confer immunity, we may be watching genetic evolution in action as a new host-parasite relationship is becoming established.
Within the bee population, there exist various forms of each gene (each form is called an allele), just as there are various alleles of the pigmentation genes for different body colors. Natural selection will favor certain alleles if they are adaptive against a parasite threat. We have watched this process happen after the recent introductions of some now common parasites– tracheal mite and chalkbrood (those processes have essentially reached “equilibrium”), varroa (still in progress), and Nosema ceranae (I’m not sure how far along that relationship is). No telling about the Army’s iridovirus!
But genetic evolution proceeds at a relatively sedate pace unless most of the host population is dying from the parasite (as occurred after the introduction of tracheal mite and varroa). However, it is clear that bees are able to gain some degree of immunity much more quickly, as evidenced by the operations that have recovered from CCD and/or epidemics of any of the aforementioned parasites. (Beekeepers still intervene with treatments for varroa and nosema, but neither of those measures has any lasting effect against the parasites).
As opposed to the tedious genetic process by which bees slowly evolve resistance to a parasite, a much quicker process is via epigenetics–that is by tweaking the way in which they express their already existing genes. Epigenetic modifications allow for very rapid evolution of not only immune response and resistance to specific pathogens, but for all sorts of other adaptations (I will cover bee epigenetics more fully in the future).
Bee colonies have two sorts of “generations,” one being at the colony level (in which the daughter colony is left behind when a colony swarms), and the other being the successive rounds of brood (generations of workers, which are replacement sisters). In either case, the offspring will likely inherit the same pathogens as their parents or siblings. So it makes sense for bees to be able to pass on, or upregulate, their gained immunities to the next generation.
The queen of epigenetic science is Dr. Eva Jablonka (2009), and there are a few bee researchers who stand out in field of bee immune function (anything that they publish is worth a read!). Sadd and the Schmid-Hempels (2005) found that by injecting a queen bumblebee with bacteria, that the induced antibacterial proteins (normally only produced in response to an infection) of her offspring would be upregulated! Moret (2006) then demonstrated the same thing occurring in mealworms. The antibacterial peptides are fairly generic, although there is evidence that the transgenerational immune priming may actually be specific for the type of bacterium (Little 2003).
Little’s and Moret’s experiments were with nonsocial invertebrates, so that the epigenetic priming must have been via the egg. Bees, however, by virtue of their maternal feeding of their larvae, have another avenue by which they could transfer immune priming—via the jelly. Such priming could also be passed from bee to bee by the same mechanism. This “social transfer” of infection resistance was demonstrated by Traniello (2002) in termites.
As you might have guessed, this discussion is leading back to immunity to viruses. I have watched colonies that appeared to be on their deathbeds with Sacbrood or Deformed Wing Virus recover spontaneously and fully. Note that I said that the colony, rather than the individual bee recovered (although there is evidence that individual bees can purge an active virus infection). It is striking to me that an entire bee operation that is suffering from virus-induced collapse can recover spontaneously, given good conditions (like a plentiful nectar and pollen flow).
Dr. Joe DeRisi (2011) has been monitoring virus levels in colonies over the past year. He found that virus epidemics in colonies occur in “sporadic waves” and then largely disappear. Dave Wick (pers comm) has observed the same, using different technology. DeRisi’s collaborator, Dr. Michelle Flenniken (2010), marvels at the delicate “equilibrium” that is generally maintained in colonies between the bees and their viruses: “These cases of persistent infection illustrate an exquisite equilibrium between the host RNAi machinery and the viral RNAi suppressor, a balance that determines the pathogenic outcome of infection, virus survival, and virus spread. The virus and host mechanisms responsible for maintaining the delicate balance achieved in persistent infections in which both parties thrive is a fascinating avenue of research.”
I came across a relevant study from India (Verma 1990), where the indigenous bee, Apis cerana, had been devastated by a new strain of virus, Thai Sacbrood Virus (TSBV; also called Chinese Sacbrood in China), first reported from Thailand in 1976. TSBV spread to the entire region of the Hindu Kush Himalayas: Burma, Nepal and India. More than 95% of the colonies were killed, particularly in the temperate regions where this disease is most widespread!
The researchers fed 25 healthy survivor colonies suspensions of the virus; 3 developed severe symptoms of the disease and were destroyed. The remaining 22 colonies showed typical symptoms of the disease, and were then reared through 5 generations (each colony’s queen was replaced by a daughter of the queen) to test resistance against the disease, each generation being again fed with a freshly purified virus suspension in sugar syrup. Now here’s where it gets interesting! I’ll quote directly:
“When the symptoms of the disease appeared, they appeared within 4-10 days after feeding the virus suspension. Such symptoms appeared earlier in parent (mother) colonies than in subsequent generations… [T]he percentage of affected brood (sealed-perforated) was greater in parent than in daughter colonies, and a continuous decrease in infection was observed in subsequent generations.
“In the present investigation, recovery from infection was observed within 30 days after the appearance of disease in the colonies [compared to the initial 95% mortality]. Parent colonies took longer to recover from infection than daughter colonies. Similarly, [the] percentage [of diseased brood] decreased…from parent to 5th generation.
“In parent colonies, abnormal behavior such as failure to cover brood by nurse bees, tendency to abscond, increased aggressiveness and reduced ability to clean out dead brood was observed, but in subsequent generations, there was gradual improvement and colonies showed normal behaviour.
“These results suggest that some mechanism of resistance to Thai Sacbrood Virus disease exists in A. cerana. In nature, this disease had a 4-year cycle and, after this period, surviving colonies began to multiply in a normal way. ”
The astute reader may notice the similarity to the many historical bee epidemics (Underwood & vanEngelsdorp (2007). An epidemic blasts through an area, and then simply “disappears” of its own accord. Looks to me like host/parasite evolution in action!
So let’s look at what happened. The only significant possible genetic selection that could have occurred in the experiment, after the initial weeding of three colonies, would have been via the drones, which came from the experimental apiary (so I suspect that little genetic evolution took place). It appears to me that the natural immunity gained over the course of five generations was likely more due to epigenetics (although there may have also been evolution of the virus during the experimental period). Can we capitalize on this?
The benefit of breeding from survivors should be obvious, as well as intentionally challenging breeder colonies with pathogens (a la bee breeder Dan Purvis). So here’s a question: Could you help a failing colony by adding a frame of nurse bees from a nearby thriving colony? Would the nurses carry immune factors from the healthy colony to the sick one? I don’t know the answer. However, we may soon have another option…
Practical Applications
In 2007, a patent was filed for the use of dsRNA in prevention and treatment of viral infections in honeybees (Paldi 2008)(refer to my recent articles for an explanation of RNAi). I’ve previously written about the field trial that I ran of the product (Sick Bees Part 2), in which we created the collapse of a yard of bees via an inoculation with a virus (largely IAPV/KBV) cocktail. The results of the previous year’s trials were recently published (Hunter, et al 2010).
The authors conclude: “IAPV specific dsRNA (Remebee-I) was used successfully to prevent bees from succumbing to infection from IAPV. The results further demonstrate the possibility to produce targeted treatments for bee pathogenic diseases. These field results demonstrate the successful application of dsRNA as a viable treatment to solve a real world problem, which may further lead to concerted efforts to utilize this ubiquitous natural mechanism, RNAi, for the benefit of the bees, beekeepers, and hopefully to other applications in agriculture and veterinary health.”
What is RemebeeTm and How Does it Work?
Remebee is simply double-stranded copies of relatively long (~800 “base pairs”) portions of the Israeli Acute Paralysis virus RNA strand, from two different regions (covering, in total, about a tenth of the virus genome). When fed in sugar syrup, it kick starts the natural antivirus response in bees, similar to the way in which vaccines work in humans—the vaccine doesn’t actually kill anything, it only tells the immune system what to attack.
When the bees ingest Remebee, it is absorbed into their gut cells, and within those, the Dicer enzyme chops up the 800 bp strand into many different short (~25 bp) dsRNA’s, (please refer to Sick Bees Part 4). It is these secondary short sections that then produce the actual bee antiviral response in a completely natural process. In fact, it appears that the preexisting presence of a low-level virus infection improves the efficacy of the treatment!
Natural Amplification and Spread
At this point, the gut cells may gain immunity to the virus, but how about the rest of the cells in the bee’s body? It has been recently demonstrated (Saleh 2009: Lipardi 2009) that insects have mechanisms to amplify the initial RNA interference response, and then spread it systemically throughout their bodies. Even an exposure to a small amount of a dsRNA product like Remebee can elicit a full systemic immune response! No one is quite sure how the immunity spreads from there, but my guess would be that it is via the jelly passed throughout the colony.
Is Remebee Safe?
Remebee is nothing more than natural virus sequences that would be produced in a natural virus infection. It’s been tested on over 10,000 colonies, and does not seem to have any negative side effects. However, keep in mind that a monthly treatment appears to be more effective than a weekly treatment, as weekly treatment may “saturate” the antiviral mechanism. There is still much to learn!
Does it work?
Beeologics has tested the product in thousands of hives in a number of countries (although generally without the intentional inoculation of viruses). It appears to be safe, and to a measureable extent, effective. Remebee is currently available for use by any beekeeper in the U.S. who wants to test it under an Investigational Use Permit. It appears that treatment may suppress virus reproduction enough to allow bees to live longer as adults. By simply adding a few days to the average life of a forager, the colony population and honey production may increase substantially.
Results from my California Trial (continued)
I never finished sharing the results of my California trial (run concurrently with another trial in Florida). To their credit, Beeologics approached me and the monitor for the trial, Dr. Eric Mussen, as two totally objective parties that are in general skeptical of new products (the Florida trial was run by USDA virologist Dr. Wayne Hunter). Beeologics also gave me permission to publish all results—good, bad, or indifferent.
Disclaimer: I am not a pitchman for the product, and have no financial interest in the company. I feel that it is fortuitous that I was offered the chance to run the trial, as it gave me great insight into the processes leading to colony collapses. I’m as curious as anyone to see how the product works in “real life” situations.
At the start of the trial (prior to feeding any Remebee) about 3/4ths of the colonies tested positive for IAPV, although usually at very low levels. Nearly all tested positive after inoculation. Unfortunately, the protocol required only this confirmation of virus titers, so I was not able to track the actual prevalence of viruses in the collapsing colonies. However, tests were run to determine the levels of siRNA in a sampling of hives at, and shortly after, virus inoculation.
Of interest is that in the untreated colonies in the Florida trial, those that already had naturally high levels of siRNA (indicating that their antiviral immune response was already in action) survived to the end of the trial, whereas those that did not crashed. In both trials, siRNAs levels increased dramatically in the Remebee-treated colonies two weeks after virus inoculation, but remained at much lower levels in the control (non Remebee) group. This indicates that the feeding of Remebee indeed worked to ramp up the antiviral immune response.
After the midwinter virus inoculation, the colonies began to crash within two weeks, and most continued to dwindle and/or suddenly collapse for the duration of the trial (until July). Of note is that I observed an above average number of hives going queenless during the trial, indicating that such failure to supersede may be related to virus infection. By comparison, similar collapses were not observed in the rest of my operation in the same county (the test yard was isolated from them).
I was completely blinded as to treatment throughout the trial, but it was not hard to guess which of the three treatments was the control, as the six groups of “green coded” colonies were obviously hit harder than the rest (this is a beauty of being blinded, since as an investigator you can then look impartially for any treatment effects).
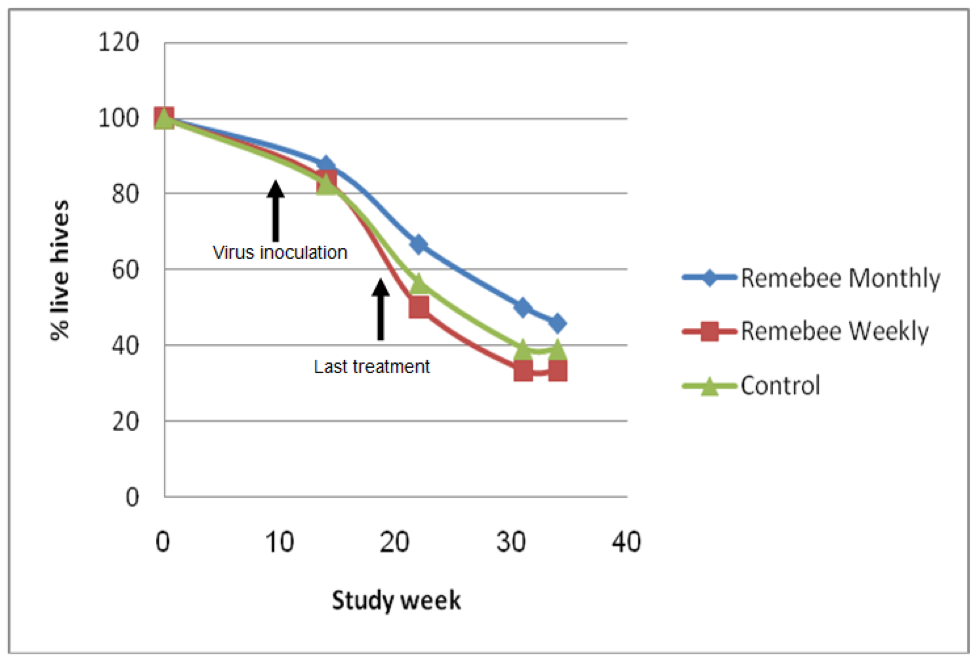
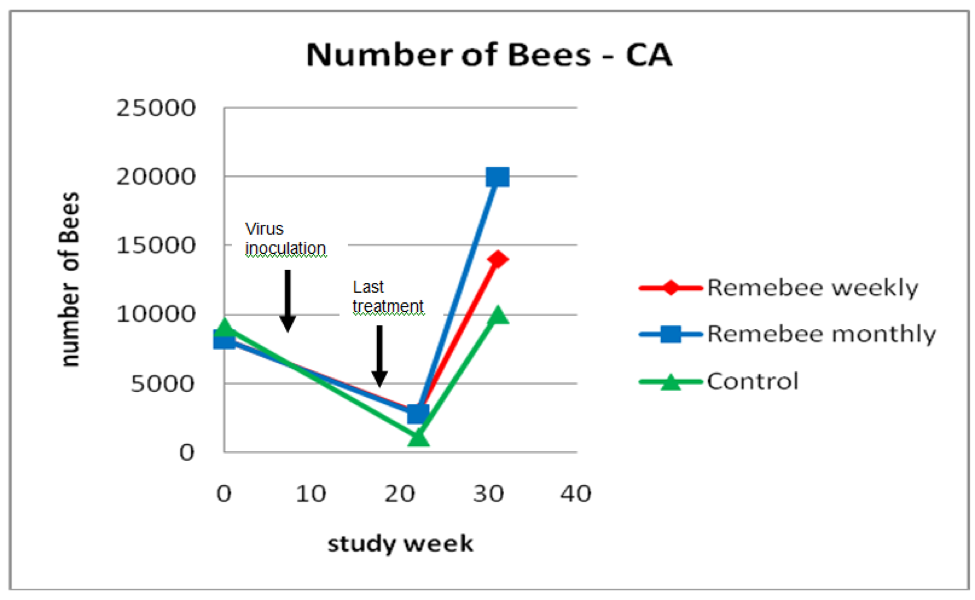
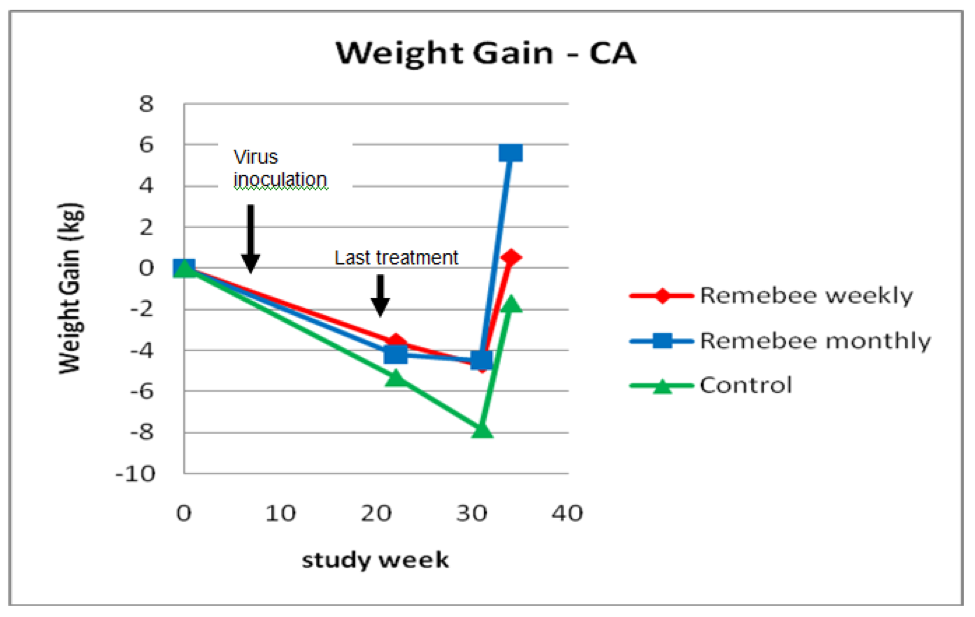
As far as survivability, colony strength, and honey production, there appeared to be a significant difference between the control and monthy treatment groups (Figures 1, 2, and 3).
Figure 1. Colony survivability. Treatments began at Week 0, all three groups were inoculated with virus at Week 9. There was little difference in colony survivability between the groups. However, the colonies were relatively small, and got hit by snow shortly after virus inoculation, which appeared to knock the snot out of them (that is the proper scientific term). There was no colony mortality until after the virus inoculation. The test yard then suffered snowstorms every week through the end of May, which was unusually stressful to the bees. Initial graphs courtesy Nitzan Paldi.
Figure 2. Results for strength of the surviving colonies. The hives treated with Remebee appeared to recover better. Note that the lines merely connect the points, and are not necessarily representative of the actual populations between the points.
Figure 3. Weight gain or loss. The trial ran from Sept 30 until July 2, but we had some snow every week in spring until week 31 (May 20), after which the few surviving colonies put on weight (I needed to take weights on July 2 before the strong colonies swarmed). Note that the final weights are only for the approximately 40% of colonies that survived to that point.
The above graphs suggest that feeding of Remebee was of benefit, but did not save this apiary from decimation, likely due to the strong virus inoculation and miserable weather conditions (cold, wet, snowy). I would guardedly say that Remebee appears to be of benefit, especially when the results of the other trials are also included in the analysis.
How About Nosema ceranae?
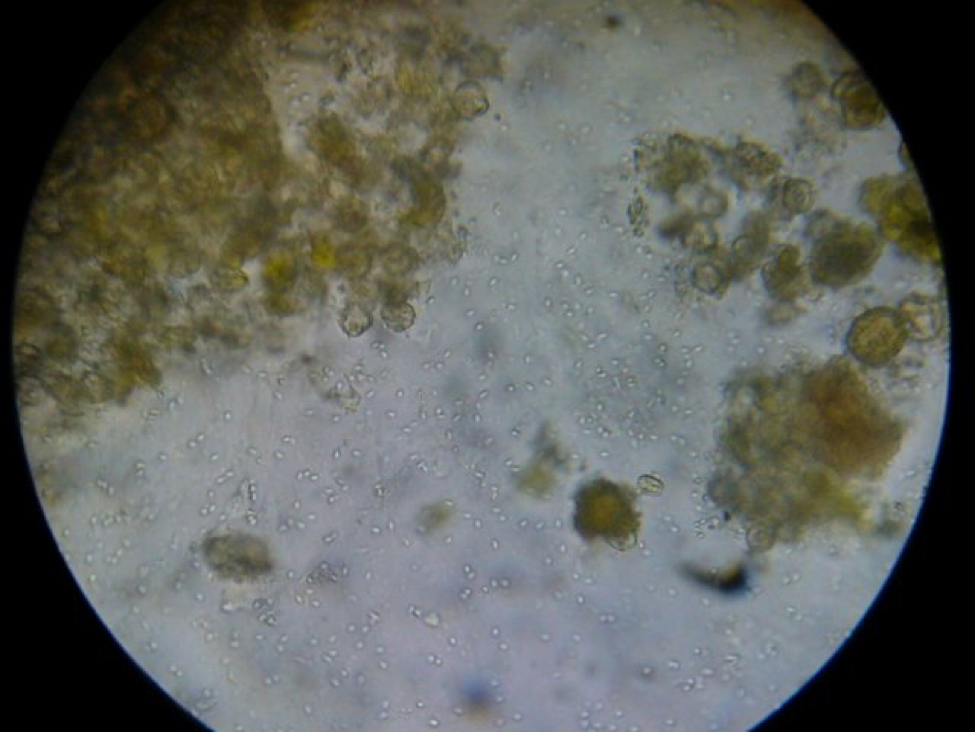
The trial was run with colonies that were infected with N. ceranae to some degree. At the end of the trial, I took samples of house bees from the remaining collapsing colonies as well as the few thriving ones, and squashed the bees one at a time do look for nosema spores. In general, the thriving colonies had little or no nosema, but in some (but not all) of the collapsing colonies, about half of the nurse bees were badly infected (Figure 4). This is something that I had not previously observed, as normally N. ceranae spores don’t show up in house bees until they’ve aged past the nurse bee stage. This observation is similar to that reported by Higes (2008) in the collapse of colonies in Spain. However, I cannot tell you whether nosema was the cause of the problem, or whether it was simply an opportunistic parasite in collapsing colonies (I still have some spore counts of samples to run, which may shed more light on any possible nosema/virus synergy).
Figure 3. A “gut squash” of a house bee from a collapsing colony. I am assuming that this was a nurse bee due to the gut full of pollen grains. Note the hundreds of nosema spores in this field of view. Nosema ceranae spores are not normally found in nurse bees, but in some of the collapsing colonies in my trial a substantial proportion had severe infections, as observed by Dr. Mariano Higes in Spain.
The Future of RNAi
Beeologics is currently developing “Remebee Pro,” which is designed to be effective against several viruses simultaneously. In collaboration with Dr. Jay Evans and the team from USDA-ARS in Beltsville, Beeologics also successfully used RNAi to suppress Nosema ceranae (Paldi 2010). Recently, Dr. Alan Bowman in Scotland used RNAi against varroa (Campbell 2010). Don’t hold your breath on this one, since there are still major hurdles ahead (we owe a note of thanks to Dr Jay Evans of the Beltsville Lab for his foresight in sequencing the varroa genome, which allowed for this sort of application).
Transgenic Bees?
A limitation of RNAi for parasite control is that it is relatively transient. However, scientists have figured out how to use a virus vector to insert siRNA sequences into the host genome (Kang 2008). Such insertions are stable and can be passed to future generations (Kennerdell 2000). There is great interested in inserting RNAi into mosquito populations in order to control the malaria parasite (Brown 2003, Perrimon 2010), and no particular hurdle against doing the same with honey bees!
What? Genetically-modified insects!, you may ask. Remember from last month’s article that this is a totally natural process that has been done by viruses since life began, and as a result, every human being is also a GMO! I don’t want to get into the GMO debate, but there is nothing to stop someone from “helping along” bee evolution to produce bees resistant to current virus strains, nosema, or even specific pesticides (Pesticide-Ready Bees from Monsanto). Yes, this makes me as uncomfortable as it does you…
Dang, I’m out of space, so I will have to continue next month with bee/virus evolution, and the role of varroa.
References
Anthony E. Brown, AE (2003) Stable and heritable gene silencing in the malaria vector Anopheles stephensi. Nucleic Acids Research 31(15): e85.
Campbell, EW , GE Budge and AS Bowman (2010) Gene-knockdown in the honey bee mite Varroa destructor by a non-invasive approach: studies on a glutathione S-transferase. http://www.parasitesandvectors.com/content/3/1/73
DeRisi, J (2011) Presentation at the National Beekeepers Convention, Galveston 2011
Flenniken, M, et al (2010) The Antiviral Role of RNA Interference. In Insect Virology, Caister Academic Press.
Higes, M, R Martín-Hernández, C Botías, E Garrido-Bailón, et al. (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10: 2659–2669.
Hunter W, Ellis J, vanEngelsdorp D, Hayes J, Westervelt D, et al. (2010) Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog 6(12): e1001160.
Jablonka, E, et al (2009) Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. The Quarterly Review Of Biology 84(2): 131-176. http://compgen.unc.edu/wiki/images/d/df/JablonkaQtrRevBio2009.pdf
Kang, S and YS Hong (2008) RNA Interference in Infectious Tropical Diseases. Korean J Parasitol 46(1): 1-15.
Kennerdell, J & Richard W. Carthew (2000) Heritable gene silencing in Drosophila using double-stranded RNA. Nature Biotechnology 18, 896 – 898.
Lipardi, C and BM Paterson (2009) Identification of an RNA-dependent RNA polymerase in Drosophila involved in RNAi and transposon suppression. PNAS 106(37): 15645–15650.
Little, TJ, et al (2003) Maternal transfer of strain-specific immunity in an invertebrate. Current Biology 13: 489–492.
Moret, Y (2006) ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealwormbeetle, Tenebrio molitor. Proc. R. Soc. B 273: 1399–1405.
Paldi, N & G Yarden (2009) Compositions for conferring tolerance to viral disease in social insects, and the use thereof. US Patent application 20090118214.
Paldi, N, et al, (2010) Effective gene silencing of a microsporidian parasite associated with honey bee (Apis mellifera) colony declines. Appl. Environ. Microbiol 76(17): 5960-5964.
Perrimon, N, Jian-Quan Ni and Lizabeth Perkins (2010) In vivo RNAi: Today and Tomorrow Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a003640
Sadd, B. M., Kleinlogel, Y., Schmid-Hempel, R. & Schmid- Hempel, P. 2005 Trans-generational immune priming in a social insect. Biol. Lett. 1: 386–388.
Saleh, M-C, et al (2009) Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature 458: 346-350.
Traniello, JFA, Rebeca B. Rosengaus, and Keely Savoie (2002) The development of immunity in a social insect: Evidence for the group facilitation of disease resistance. PNAS 99 (10): 6838.
Underwood, RM and D vanEngelsdorp (2007) Colony collapse disorder: Have we seen this before? http://ento.psu.edu/directory/duv2/underwood_vanEngelsdorp_2007.pdf
Verma, LR, BS Rana, S Verma (1990) Observations on Apis cerana colonies surviving from Thai Sacbrood Virus infestation. Apidologie 21: 169-174.